The Basic Principle of AAS
In order to determine elements in a base material, samples are atomized in front of a source of light. Sources of light are usually line sources (hollow cathode lamps) that radiate light in various defined wave lengths. Conventional AAS devices require that the corresponding light source be used for the element to be defined. In contrast, modern high-resolution continuum source AAS devices (HR-CS AAS) are equipped with one xenon short arc lamp, which can radiate light across the entire relevant wavelength spectrum and therefore do not need to be exchanged.
The atomization of the analyte in the sample into individual atoms is carried out with the help of a gas flame (flame AAS) or an electrically heated graphite furnace (GF AAS). For flame AAS, the sample solution is nebulized and carried into the flame, while for GF AAS the solution is put in the graphite furnace first and atomized there through high temperatures.
The atomization of the sample generates an atom cloud that absorbs and debilitates the radiated light, causing it to lose intensity (absorption). Then, the intensity of the light in relation to the element-specific wavelength before and after the atomization is measured and compared. An increased concentration of an element in a sample leads to increased absorption. This absorption signal – meaning the decrease in intensity of the light compared to the radiated intensity – can be measured, thus determining the content of the analyte in the sample. For the analysis, standard solutions with known element concentrations are measured and used to generate calibration curves in order to determine the concentration of the element in the sample to be analyzed.
UV Vis spectroscopy is also qualitatively useful in some more specialized research. Tracking changes in the wavelength corresponding to the peak absorbance is useful in examining specific structural protein changes and in determining battery composition.Shifts in peak absorbance wavelengths can also be useful in more modern applications such as characterization of very small nanoparticles. The applications of this technique are varied and seemingly endless.
Key components of a gas chromatograph
Atomic absorption spectrometers use the absorption of light to measure the concentration of gas phase atoms. The light that is focused into the flame is produced by a hollow cathode lamp, inside which is the sample and an anode. A high voltage is passed between the cathode and anode and the metal atoms are excited into producing light with a certain emission spectrum.
An atom consists of a core containing neutrons and protons. It also has a surrounding number of electrons, which are bound to the core at different energy levels. When an electron makes a transition from a particular energy level of an atom to a lower energy level, a photon of energy is released, which is equivalent in energy to the reduced level for the electron. The photon forms an atomic spectral line.
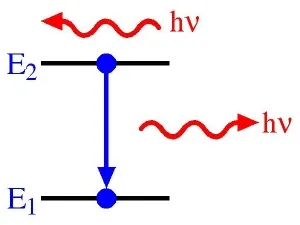
The frequency (v) at which the spectral line occurs is related to the energy (E) by Planck’s law; E = hv, where h is Planck’s constant. The atomic radiation produced can be characterised by both emission and an absorption coefficients. As the quantity of energy put into the flame is known and the quantity emitted can be detected, it is possible to calculate the concentration of the element present.
Metals that can be Detected by Atomic Absorption Spectrophotometer
This method can be used to detect metals such as:
- Aluminium Al
- Antimony Sb
- Arsenic As
- Beryllium Be
- Barium Ba
- Calcium Ca
- Chromium Cr
- Cadmium Cd
- Cobalt Co
- Copper Cu
- Gallium Ga
- Hafnium Hf
- Indium In
- Iron Fe
- Lead Pb
- Lithium Li
- Magnesium Mg
- Manganese Mn
- Mercury Hg
- Molybdenum MoNickel Ni
- Niobium Nb
- Ruthenium Ru
- Tin Sn
- Tungsten W
- Vanadium V
- Zinc Zn
- Zirconium Zr
